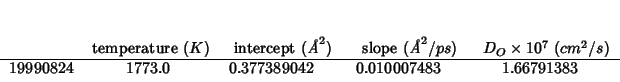


Next: 19990825: with a different
Up: MD work on
Previous: 19990823: who is who:
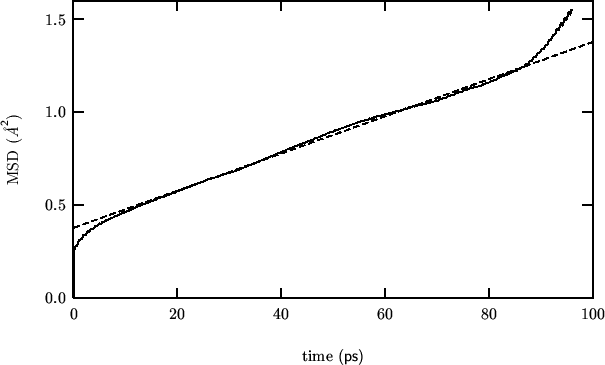
19990824:
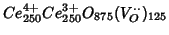
This is a calculation on pure ceria, to be compared with another one
(still to be done) on the mixed system:
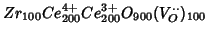 ,
which has the same reduction degree.
,
which has the same reduction degree.
To evidence any effect of zirconia addition on the oxygen diffusion
coefficient.
Based on Vegard's law, I set a lattice constant of
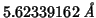 , derived from:
, derived from:
with:
and
GULP optimization gives:
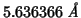 .
.
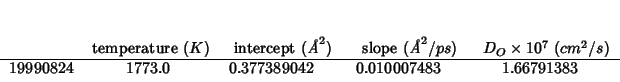
![]() , derived from:
, derived from:
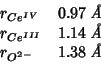
![]() .
.